Growing a leg like a lobster, could we do that too?
Lobsters can grow back lost limbs. If nature has developed that gift once, could we recreate it?
This is a question that has puzzled scientists since the 1950s, when stem cells were first made from human skin cells. This was a revolutionary step because stem cells can be used to make all kinds of cells and tissues. The techniques have been further developed in recent decades, and in the near future this regenerative medicine may offer solutions for bone disorders, organ diseases and blood diseases, among other things. For many of these conditions, there is currently only treatment for the symptoms, but not for curing the disease. Regenerative medicine will be the future for these select groups of conditions.
Reprogramming differentiated cells
In the 1950's researchers mainly used human embryonic stem cells. Stem cells from early human embryos were used to obtain the desired differentiated cell. It was also investigated whether this could be researched in a more ethical way: using a fertilized egg was a step too far for many people. Nowadays other methods are used which make it possible to reprogram a differentiated cell (for example heart or liver cells) into induced pluripotent stem cells (iPSCs) . These cells can differentiate into a desired organ or tissue. The patient's body cells can be used to form the iPSCs and apply them therapeutically. As these cells are genetically identical, they will not be rejected by the body. Currently, mainly skin cells from the patients are used to make iPSCs. However, it is not yet known what the exact mechanism in the cells is. In addition, the process is not very efficient as less than 1% of the cells are reprogrammed. iPSCs are used in multiple areas of science: studying tissues, discovering disease profiles and testing the toxicity and effect of drugs on the target organ.
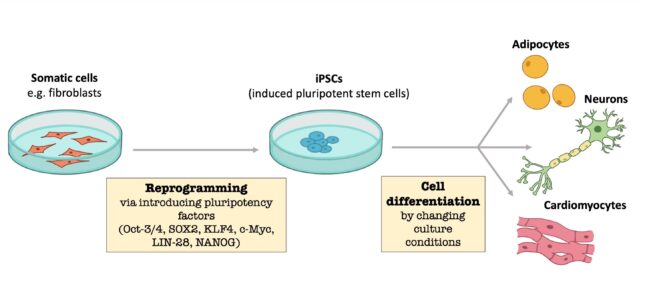
Regenerative medicine in practice
Doctors and researchers at the Leiden University Medical Center (LUMC) are currently applying regenerative medicine to diseases for which no good alternative treatment has yet been found. An example is Duchenne muscular dystrophy (DMD), a hereditary muscle disease that mainly occurs in boys. There is currently no good treatment for DMD, so children are given medication to relieve their symptoms. However, these drugs are very toxic and cause many side effects, which often means that treatment with these drugs is stopped. The LUMC is currently investigating an 'exon skip therapy', in which the abnormal genetic code is restored. As a result, the previously malfunctioning proteins will function again.
There are more types of treatments for heart disease, including lifestyle changes, medications, or surgery. Regenerative medicine offers the opportunity to treat heart diseases even better. For example, cardiac arrhythmias can be repaired by building photosensitive ion channels in the heart muscle cells. The electrical activity can be determined with the help of a light pulse, so that a healthy heart rhythm can take place again. Another goal is to generate better heart valves to replace calcified and worn out heart valves. A method is now being explored to decellularize diseased heart valves (remove the diseased cells from the heart valve, ed.) and surround them with healthy tissue prior to transplantation. With this, the researchers hope that the heart valves will last longer.
Our kidneys have an important function in our body: the filtering of our blood. This is a dangerous task as kidneys are frequently exposed to toxic substances. It is not yet possible to recover from chronic kidney failure; the only treatment at the moment is kidney transplantation. However, the queues are very long and many patients do not get their turn. Regenerative medicine offers the possibility to reduce these queues and the chance of rejection of the donor organ. After a kidney transplant, patients are treated with MSCs (mesenchymal stromal cells), a type of stem cells that have a strong anti-inflammatory effect. As a result, the donor organ can last longer and the patients need to take less immunosuppressive drugs. In addition, it is currently being investigated whether the donor kidneys can be revitalized with MSCs as this will stimulate regeneration of kidney tissue. In addition, the ultimate goal is to cure kidney patients without having to take drugs for the rest of their lives; this is only possible if the body's own kidneys are grown.
A milestone in the Netherlands
Very recently, researchers at the LUMC have made a debut in the field of stem cell therapy. After 15 years, a stem cell therapy has been developed to treat the rare congenital immune disease Severe Combined Immunodeficiency (SCID) in newborns. This is a milestone because for the first time stem cell therapy is being applied to patients in the Netherlands. Children with SCID are born without a sufficient amount of immune cells, which means that they do not live to be older than 1 year without treatment. Finding stem cell donors that fully match the children's HLA (certain proteins that are responsible for the regulation of the immune system, ed.) appears to be difficult, as a result of which patients are matched with donors whose HLA only partially matches. Stem cell therapy solves this problem, because the newborn is his or her own donor. Stem cells are collected and sent to the LUMC. Here the mutation in the stem cells is changed, after which the "corrected" stem cells are transfused back into the newborn. According to professor of stem cell biology Frank Staal and professor of pediatrics and stem cell transplantation Arjan Lankester, this therapy will open doors for the treatment of other rare diseases.
As science has shown countless times, the developments in regenerative medicine also show that nature can serve as an inspiration for impressive human developments. Lobsters can regrow lost body parts, so why not try to make that possible in humans too? Seventy years ago, this profession was still in its infancy, and now we see the first patients being treated with it. And who knows, the coming decades will show many more possibilities to replace diseased body parts with this elegant technique.
Literature:
- Govind, C. K., Pearce, J., & Potter, D. J. (1988, december). Neural attrition following limb loss and regeneration in juvenile lobsters. PubMed. https://doi.org/10.1002/neu.48...
- Lau, W., Clevers, H., Strien, M., Burm, S., & Hol, E. (2016, june 22). See: Gekweekte mini-organen. NEMOKennislink (Dutch). https://www.nemokennislink.nl/...
- Pittenger, M. F., Discher, D. E., Péault, B. M., Phinney, D. G., Hare, J. M., & Caplan, A. I. (2019, 2 december). Mesenchymal stem cell perspective: cell biology to clinical progress. npj Regenerative Medicine. https://www.nature.com/article...
- Roelen, B. (2016, june 22). Geïnduceerde pluripotente stamcellen. NEMOKennislink (Dutch). https://www.nemokennislink.nl/.../geinduceerde-pluripotente-stamcellen/
- Spierziekten Nederland: Duchenne spierdystrofie (DMD). (z.d.). Spierziekten Nederland (Dutch). Consulted on Februari 8, 2021 https://www.spierziekten.nl/ov...
- UMC Utrecht. (z.d.). Regeneratieve geneeskunde en stamcellen (Dutch). Consulted on Februari 8, 2021, van https://www.umcutrecht.nl/nl/z...
- Rathenau Instituut. (2018, 8 mei). Regeneratieve geneeskunde: behandeling van de toekomst? (Dutch) https://www.rathenau.nl/nl/maa...


0 Comments
Add a comment