The limitless future of RNA
RNA has catapulted to the forefront of the pharmaceutical sciences. Elegant and effective, RNA can target and utilize the inner workings of our cells to protect us from a wide range of diseases. What can we expect from RNA-based therapies in the foreseeable future?
While people all over the Netherlands are rolling up their sleeves for their booster shot, it dawns on many that innovations in the pharmaceutical industry can be of great service to mankind in times of need. Out of the more than 150 coronavaccines that were in development last year, the RNA-vaccines – the vaccines of Pfizer-BioNTech and Moderna – have achieved the greatest successes. Research into RNA has been under way for several decades, with more setbacks than you can count, but thanks to a confluence of events, scientists could finally deliver a safe and effective product at the end of 2020. The pandemic set it all in motion.
In time, the coronavirus may fade away, but RNA-vaccines are here to stay. Prompted by its vast potential, researchers have already been looking for new ways to use RNA, for instance to treat hereditary diseases, or even to wield the immune system against cancer. How broad can RNA be utilized? Is it indeed the golden weapon with which we can eventually defeat all disease and ailment from the world?
Shopping list
RNA stands for ribonucleic acid. It is a large molecule that is produced in all living cells. RNA can be regarded as the shopping list with which a cell makes protein. A small stretch of DNA – our genetic material – is transcribed to a copy inside the cell nucleus. This copy (the mRNA) will go to the cytoplasm and act as a recipe for the synthesis of proteins, the building materials and catalysts of the cell. A liver cell's shopping list is completely different from that of a muscle cell or a brain cell. Using the code in the mRNA, a protein will be produced that carries out a specific biological function, ultimately defining the behavior of the cell in which it is made. Now, with RNA-vaccines and RNA-therapeutics, we are simply adding an extra shopping list to all this wonderful machinery.
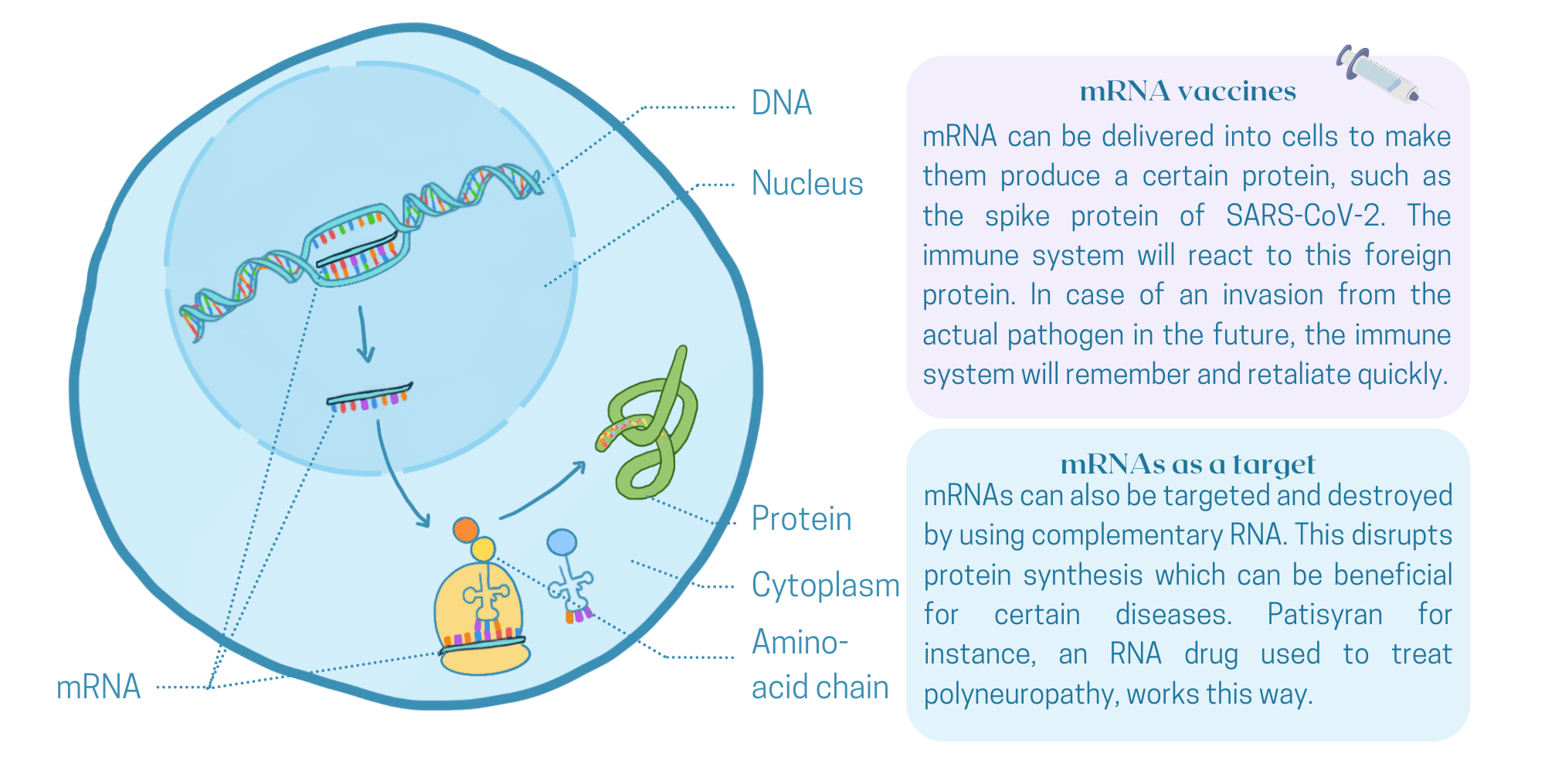
Many scientists believe that RNA-therapeutics will have a huge impact on the healthcare sector of the twenty-first century. Some even call it an RNAissance: the idea to use RNA has been around for some time, but only recently pharmaceutical companies appear to have been convinced of its power. In the last five years, more than a hundred RNA-based therapeutics have been studied in clinical trials. It is only a matter of time until new RNA-drugs will make it to the market. Neuromuscular disorders, heart diseases and even some forms of cancer are currently leading in the pipeline, as reviewed recently in the Dutch Journal of Medicine.
Perseverance
The foundations of RNA-based therapies have been established by the Hungarian biochemist Katalin Karikó. In collaboration with the American immunologist Drew Weissman, she found out that RNA can be modified in such a way that it loses its notorious inflammatory properties. The trick was chemically changing certain nucleotides (building blocks of the RNA-molecule), allowing the RNA to be injected safely.
Her dedication and perseverance, in spite of the setbacks and turndowns she encountered along the way, have inspired many. To this day, she is committed to optimizing RNA that produces a therapeutic protein inside the body – and this can be anything, even a protein that triggers the immune system to fight cancer cells. Theoretically, it would allow researchers to tackle an almost unlimited range of diseases in a remarkably simple and elegant way. It has led some scientists to speculate RNA could become a central asset in the future pharmaceutical industry.


Concerted efforts
The impact of awakening RNA technologies is also become apparant in Leiden. Leo Visser, head of the Department of Infectious Diseases at the LUMC, is impressed with the pace with which the RNA-vaccines are changing the pharmaceutical market. “The combination of political will, financial possibilities and scientific collaboration enable us to take enormous steps.” Meanwhile, knowledge that is amassed a bit more quietly over the years, is crucial too. “Without the insights of Katalin Karikó, we would not have had the vaccines.”
Visser is studying vaccination and immune responses against tropical pathogens, in particular against yellow fever and rabies viruses. His team is interested in finding alternative administration routes, to make vaccines last in times of scarcity. “Reaching as many people as possible, with as little vaccine as possible”, is the ultimate aim. One way to do this, is to inject not in the muscle, but in the skin. “Exactly in the layer of skin where there are a lot of antigen presenting cells. Then, you only need a fraction of the vaccine, but since you address these immune cells directly, the resulting protection is still high.”
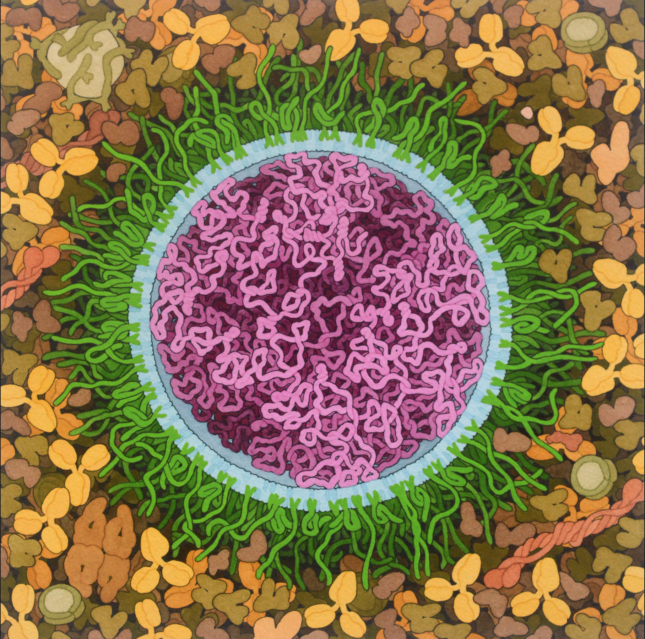
So why is RNA so interesting for vaccine purposes? That is because of the many advantages. The molecule is relatively simple to produce (even on a large scale), it is easily adaptable when new variants unexpectedly arise, and in addition, it is remarkably easy to personalize.
Visser: “With RNA you have the possibility, for instance, to engineer a multiplex vaccine that expresses multiple antigens (instead of just one spike-protein that the virus easily mutates, red.). Such vaccines are suitable against many variants of one pathogen species, which is an ancient aim in the vaccine industry.” Of course, there is still a lot of work to do and challenges to overcome. “Making the RNA more stable, targeting them to certain cells in the body, storing them more economically, there is still a lot to solve.”
Antisense
Obviously, RNA is more than just a vaccine-ingredient. First of all, it is the ‘interpreter of all of our genes’, and plays a fundamental role in health and disease. Mutations in the genetic material can sometimes give rise to ‘miscoded’ RNA-molecules. And these can have disastrous effects on our health.
Willeke van Roon-Mom, professor of human genetics and head of the Dutch Center for RNA Therapeutics (DCRT) in Leiden, is involved in the development of specific RNA-therapeutics against such diseases. Her career is centered around hereditary brain diseases caused by toxic proteins. “What we do here is finding ways to break down RNA-molecules that code for those proteins. To tackle them, we use the so-called antisense oligonucleotides.” By cooperating closely with patients, clinicians and companies, the DCRT dedicates its efforts to the development of therapeutics against very rare genetic conditions. “Conditions that may have a smaller chance at the bigger pharmaceutical companies”, she explains.
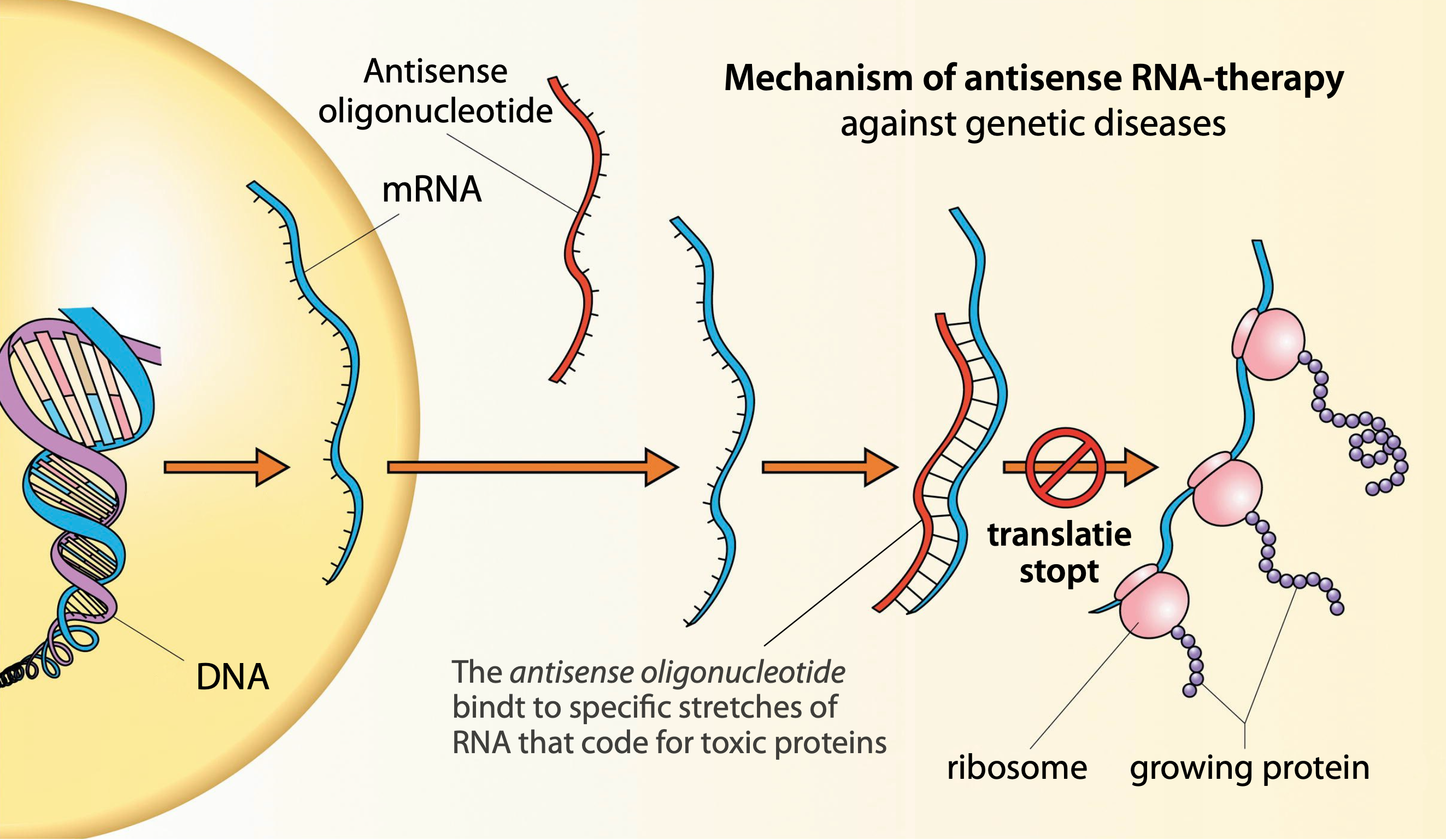
Indeed, this is something completely distinct from RNA-vaccines: here, RNA is constructed so it specifically binds to mutated RNA-molecules, breaking them down, and thereby preventing the expression of toxic proteins. Van Roon: “A fundamental problem is that we can alleviate or slow down an illness through this process, but we cannot cure it. To achieve that, you must look a step deeper, to DNA-modification techniques.”
That doesn’t alter the fact that at this moment there are many RNA-based techniques in use that can significantly improve the health of patients. “The field is very wide. Splice-modulation, downregulation, you name it. I expect that we will take big steps in all of these applications, and I hope that we continue to expand our knowledge in the coming years about how to increase efficiency further, while keeping side effects low. Even clinical trials that turn out less successful, can give us invaluable insights how to improve our products.”



0 Comments
Add a comment