The Unique Spark: Why Fire Needs Earth
Did you know that fire, as we think of it, can only exist on Earth? The atmospheric candlelight on your table, the bonfire you sat around with friends the other night - it is all only possible in the conditions we find here on Earth.
The 3 elements of fire
To discover why fire is unique to Earth, we have to look into the chemistry behind fire and Earth’s unique characteristics.
Fire is a chemical reaction known as combustion, which requires three essential components: fuel, oxygen, and heat1 – collectively referred to as the “fire triangle”. Only organic materials, which are composed of carbon atoms, can serve as fuel for combustion. These include substances like plants and fossil fuels, which are abundant on Earth. For the reaction to begin, fuel must combine with oxygen. Earth's atmosphere, containing approximately 21% oxygen2, exceeds the minimum amount of oxygen needed to sustain fire. Heat is the third and final component of combustion, as only gases can actively participate in the process. It triggers the release of these gases from the fuel, enabling the reaction to occur. On Earth, naturally occurring phenomena such as lightning, volcanic activity, and friction can act as fire igniters.
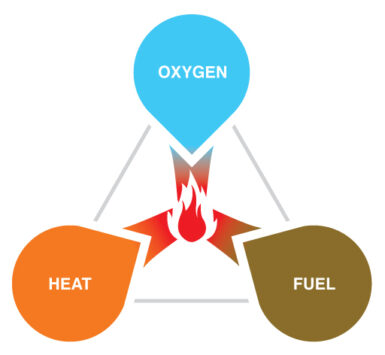
The fire triangle can be understood more easily by observing a candle. Striking a match demonstrates how friction generates heat, creating a flame that initiates combustion on the candle. The fuel used for candle making has evolved over time3; in ancient Egypt, people used papyrus reeds coated with animal fat as torches. Modern candles, however, are typically made from paraffin wax (derived from petroleum) or, less commonly, beeswax, palm wax or soy wax.. These materials are all hydrocarbons and therefore organic fuels. Lastly, the role of oxygen becomes apparent when it is removed; cover your candle with a glass and the flame will extinguish due to a lack of oxygen. To summarise, once heat initiates combustion, the flame will only continue to burn as long as wax (fuel) and oxygen are present. Therefore, all three components are necessary to sustain fire.
Comparison to other planets and moons
While the individual components of fire do exist on other planets, the precise combination of heat, organic material, and the right amount of oxygen is exceptionally rare.
Nevertheless, organic molecules are not only abundant on Earth but also commonly found on other objects4 in the solar system. It is important to emphasize again, however, that "organic" does not necessarily imply life; rather, it simply refers to carbon-containing molecules. For example, Saturn’s largest moon, Titan, is believed to have lakes of pure methane (CH4)5 on its surface, and other organic-rich material in its atmosphere. On another one of Saturn’s moons, Enceladus, organic molecules were found in its plumes6, which are streams of gas and icy particles ejected from beneath its surface into space. Meteorites, comets and asteroids are also known to carry organic material. However, as well as sufficient heat, most of these environments lack the key reaction partner for combustion: oxygen.

When it comes to the third component, oxygen, you might have heard that Mercury’s atmosphere7 also contains oxygen, even a high percentage of around 42% - compared to Earth’s 21%. While this is true, Mercury’s atmosphere is extremely tenuous and not even classified as an atmosphere, but rather as an “exosphere”. This is because its gases are constantly blown away by solar radiation and the solar wind, which is a stream of charged particles such as electrons and protons. Without a stable atmosphere, Mercury cannot sustain a fire. Elsewhere in our solar system, the atmospheres of Venus and Mars are primarily composed of carbon dioxide, and the gas giants (Jupiter, Saturn, Uranus and Neptune) do not even have a solid surface. This means that our traditional fire is impossible on these planets as well.
Other planets and moons can undoubtedly host different forms of energy production, luminous phenomena, or even combustion using alternative oxidizers8 like fluorine or chlorine. However, of all the planets in our solar system and the exoplanets discovered so far, only Earth provides the right conditions for the traditional combustion that we know as fire. Until we find a planet that resembles Earth closely enough, it seems that this ’unique spark’ - the ability to sustain fire as we know it - remains a phenomenon only our world can claim, making every atmospheric candlelight and glowing bonfire a testament to Earth’s extraordinary chemistry and atmosphere.
Sources
Video inspiration: https://www.youtube.com/watch?v=tMDKeBaLWDw
- What is fire? (z.d.). Science Learning Hub. https://www.sciencelearn.org.n...
- Society, P. (2023, 11 mei). How did Earth get its oxygen? The Planetary Society. https://www.planetary.org/arti...
- Candles: What do they emit when lit? (z.d.). Office For Science And Society. https://www.mcgill.ca/oss/arti...
- Kwok, S. (2019). Organics in the solar system. Research in Astronomy And Astrophysics, 19(4), 049. https://doi.org/10.1088/1674-4...
- Organic compounds in Titan’s seas and lakes - NASA. (z.d.). NASA. https://www.nasa.gov/image-art...
- Cassini samples the icy spray of Enceladus’ water plumes. (z.d.). https://www.esa.int/Science_Ex...
- Choi, C. Q. (2023, 9 juni). Mercury: A complete guide to the closest planet to the sun. Space.com. https://www.space.com/36-mercu...
- Lunawat, R. (2023, 19 oktober). Can Fire Burn When There’s No Oxygen? ScienceABC. https://www.scienceabc.com/nat...






0 Comments
Add a comment