Water’s Wonders
Water is a tasteless, odourless, transparent liquid that does not provide us with any nutrients or energy. Nevertheless, it is vital for life on earth. But what makes water so special?
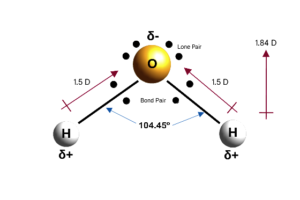
Inside the water molecule
Water has a number of properties that make it a unique element. It is the only compound that naturally occurs in all three aggregate states on earth: In solid form as ice, in liquid form as water and in gaseous form as vapour. Many of the properties of water can be traced back to its chemical structure. A water molecule consists of one oxygen atom (O) and two hydrogen atoms (H). Oxygen, being highly electronegative, exerts a strong pull on electrons, while hydrogen holds onto its electron much weaker. This results in the formation of a dipole within the water molecule: The oxygen side of the molecule becomes slightly negatively charged, drawing in the hydrogen electrons, while the hydrogen ends are slightly positively charged. Because of this polarity, water can form hydrogen bonds between the oxygen atom of one water molecule and the hydrogen atom of another water molecule. This occurs due to the positive partial charge of the hydrogen atom that interacts with a free pair of electrons on the oxygen.
The anomaly of water
Have you ever put a bottle of water in the freezer and forgotten to take it out again? The next time you opened the freezer, you probably found the bottle burst. But why is that? For most substances, the density increases when they are cooled, which in turn means that the volume decreases. However, this does not apply to water, which reaches its maximum density at around 4°C. Above and below this temperature, the density decreases progressively, which means that the water expands. This phenomenon is known as the anomaly of water. The reason why water behaves so unusually is due to its ability to form hydrogen bonds. Due to these bonds, water molecules gather in clusters, which are already formed in the liquid state. At 4°C, the clusters are arranged in such a way that they require the smallest possible volume, like neatly stacked goods on a supermarket shelf. However, if the temperature drops further, the structure changes while crystallising, which means that the water needs more space again. On the other hand, when the temperature increases, the thermal movement of the molecules ensures that the clusters dissolve and the water in turn expands. This unusual behaviour of water is responsible for the fact that ice floats on the water surface. Furthermore, it allows many aquatic creatures to survive the winter, as 4°C water has the highest density and sinks to the bottom, providing them with a liquid layer to live in.
A blue planet
In total, there are around 1.4 billion cubic kilometres of water on Earth, making it one of the most widespread molecules on our planet. However, the majority is found as saltwater in the oceans, which cover three quarters of the Earth’s surface. The oceans give our planet its blue colour. But why blue if water appears to be transparent? The reason is that water absorbs light in the range of 600 to 800 nanometres. This is the long-wave range of visible light or, in short, the colour red. Short-wave light, on the other hand, which we humans perceive as blue, is not absorbed by water, but scattered. This gives the impression that the colour of water is blue. Although the colour of our planet suggests that there is more than enough water, only around 3% of the water on earth is freshwater and therefore drinkable. However, two thirds of it are bound up in the polar ice caps and glaciers and are therefore not available to us. Another large proportion of freshwater can be found as groundwater and only a small percentage forms the rivers and lakes of our planet. So, although the incredible quantities of water on earth give our planet its blue colour, fresh water is a finite resource that should be used responsibly.

The element of life
Water is called the element of life for a reason. It fulfils many tasks in our body and operates under many different names. For example, our brain floats in what is known as cerebrospinal fluid, which protects the brain from shocks and enables transport between cells. For maintaining the right pressure within our eyes we have the aqueous humour - a fluid consisting predominantly of water. We are also dependent on water when we breathe, as our lungs need to be moist to ensure an optimal exchange of oxygen and carbon dioxide. Not to mention our cardiovascular system, which distributes blood and therefore water throughout the body. Due to its molecular properties, water functions in our body as a medium, solvent and catalyser. For example, salts that are essential for our survival are dissolved in our body water as ions and many chemical reactions require water to take place. Compared to other liquids, water can also absorb a relatively large amount of energy without heating up significantly. This means that the excess energy from chemical reactions in our cells can be absorbed by the water, thus preventing the cells from overheating.
So even if this small molecule seems very inconspicuous at first glance, water shapes our bodies as well as our planet. It plays a significant role for the functioning of the tiniest cell to entire ecosystems and is the reason why life is possible at all.
Sources
Triebskorn, R., and Wertheimer, J. (2016). Wasser als Quelle des Lebens: Eine multidisziplinäre Annäherung. 10.1007/978-3-662-46268-3






0 Comments
Add a comment